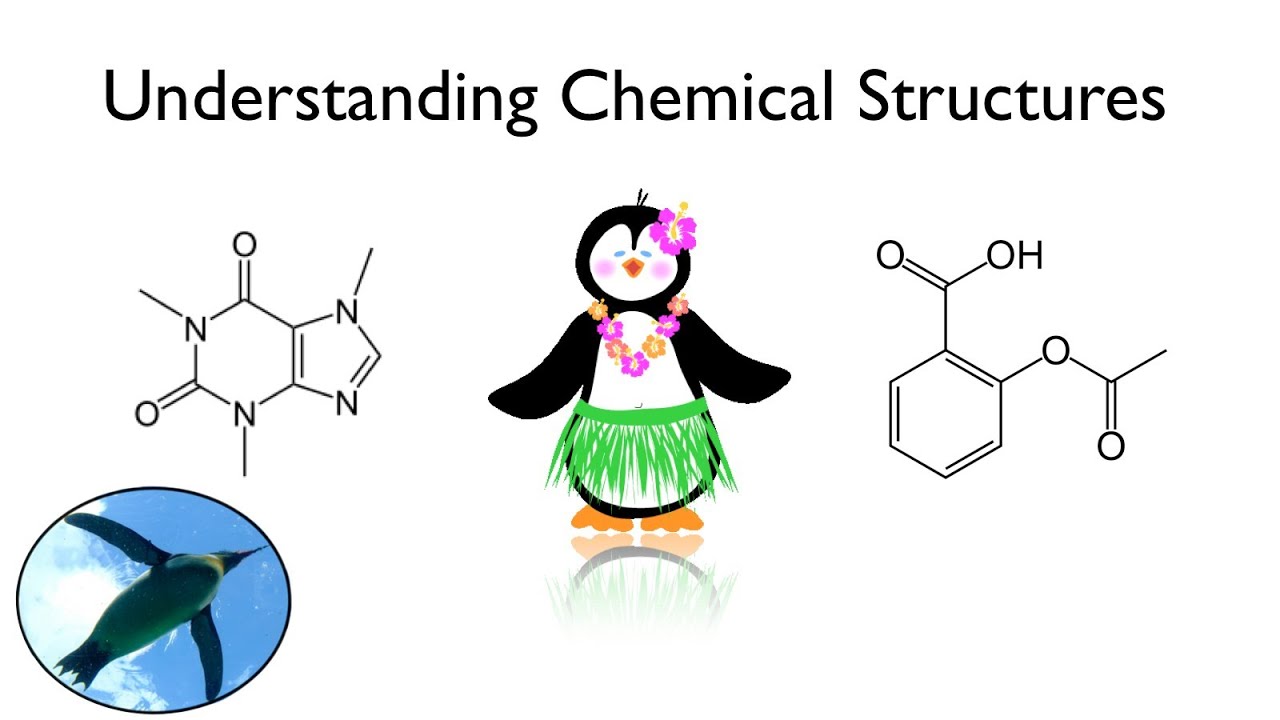
Drawings and naming organic molecules leads to mass confusion for Biology students, most of whom have not yet taken Organic Chemistry. This video will introduce you to this strange world filled with chains and rings and help you to make sense of it. If you have watched my CHEMISTRY BASICS videos I and II (links below) you are ready for what comes next! By the end of the video you will be able to look at structures for aspirin and caffeine and figure out the chemical formula for each. YES YOU WILL!!!
Links to CHEMISTRY BASICS VIDEOS
Chemistry Basics Part I:
Chemistry Basics Part II:
JOIN THE FUN all over the WEB:
SUBSCRIBE:
.:
.+:
.:
WEB:
VIDEO DETAILS:
Feel the Power of STRUCTURES
Recall: Bonding Rules are based on valence electrons
Hydrogen: happy with 1 covalent bond
Carbon: happy with 4 covalent bonds
Nitrogen: happy with 3 covalent bonds
Oxygen: happy with 2 covalent bonds
Organic chemistry codifies lots of things:
How Many Carbons?
Types of Carbon-Carbon Bonds
Let’s get drawing and understanding:
Ethanol: (structure to formula: C2H6O
Ethanol has two carbon atoms (eth-) with a single bond between them (-ane), and an attached -OH group (suffix “-ol”)
RULES:
1. Carbon lives at BENDS and ENDS
2. HYDROGENS bring carbon HAPPINESS
Let’s try another:
Caffeine (structure to formula): C8H10N4O2
And finally:
Aspirin: (structure to formula): C9H8O4