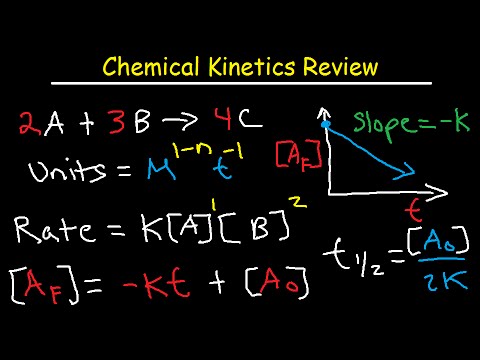
This general chemistry study guide video lecture tutorial provides an overview of chemical kinetics. It contains plenty of examples, practice problems, and conceptual questions to help you to master the course. This video is especially helpful to those taking AP chemistry in high school or general chemistry in college.
Here is a list of topics:
1. How to calculate the rate of the reaction using the change in concentration and time
2. Determining the order of a reactant and the overall order of the reaction using the method of initial rates.
3. How to determine the rate equation or rate law expression
4. Calculating the rate constant K and the units of K
5. Understanding the difference between the first order, second order, and zero order reaction.
6. Equations and formulas for zero order, first, and second order reactions
7. Half Life Formula, Initial Concentration of A and Rate constant K
8. Factors affecting reaction rate – concentration, temperature, and catalyst
9. Relationship between the rate of the reaction and the concentration
10. Rate constant K, temperature, catalyst, activation energy and potential energy diagrams
11. Forward activation energy vs reverse activation energy
12. Arrhenius Equation
13. Half Life Problems and Half Life Method
14. Collision frequency, steric factor, and frequency factor
15. Reaction Mechanism – Slow Step – Rate Determining Step
16. How To Find the Intermediate and Catalyst in a Reaction Mechanism