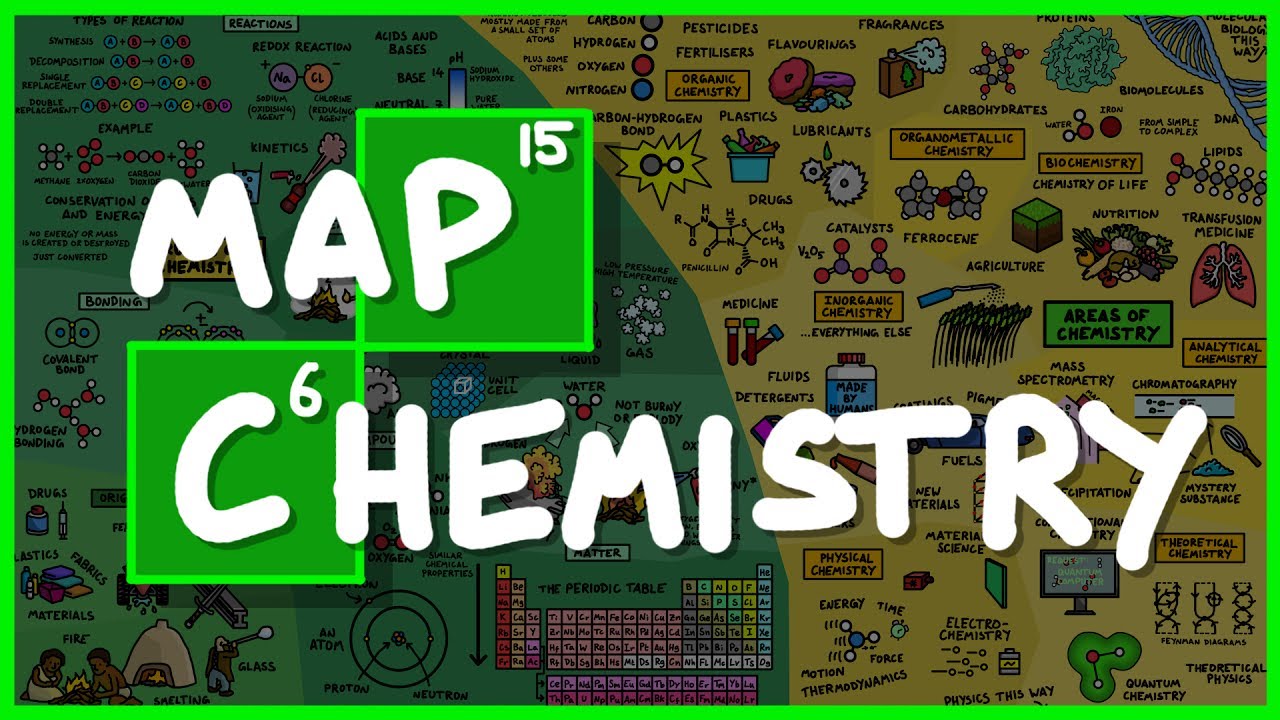
The entire field of chemistry summarised in 12mins from simple atoms to the molecules that keep you alive.This video was sponsored by The Great Courses Plus, start your free trial and help out this channel here
If you would like to buy a poster of this map, they are available here:
I have also made a version available for educational use which you can find here:
and a widescreen version:
Errata and notes:
1. I got the Oxidising Agent and the Reducing Agent the wrong way around! Sodium is the Reducing agent and Chlorine is the Oxidising agent. My confusion was that when a sodium atom looses an electron it becomes oxidised, so in my simple brain, I called it the oxidising agent. That is wrong because the agent that oxidises the sodium is the chlorine atom and so the labels are the wrong way around. Doh!
2. I drew the hydrogen H2 molecule with a double bond but it should be a single bond because they are bonded with a single covalent bond.
3. Where I have drawn carbon dioxide, the carbon should have a double bond to each of the oxygens.
4. Apparently Feynman diagrams are not that useful for theoretical chemistry, so perhaps that wasn’t the best choice for the illustration. The feedback in the comments from a real theoretical chemist is “All we deal with is shuffling around electrons, but many many many electrons, so a Feynman diagram would need to be huge but at the same time would be very very repetitive.”
5. In analytical chemistry, I should have called it distillation rather than precipitation.
6. My definition of organic chemistry being about ‘life’ is not very good. I should have said that organic chemistry looks at compounds that contain carbon. But there are some compounds in inorganic chemistry that also contain carbon, like carbon dioxide so I guess I’d also have to state that inorganic chemistry is almost everything else.
7. I said that fuels are inorganic chemistry which is misleading when I drew a car next to it. My understanding is that there are inorganic fuels that don’t contain carbon, but obviously all the fuels we are familiar with are organic. I thought a picture of a car would tie a few things together elegantly, but it ended up giving the wrong impression. That’s okay, I’m still learning! 😀
8. In inorganic chemistry, I should have stated that all natural minerals fall under inorganic chemistry so as not to be misleading, otherwise you might go way thinking that only man-made substances fall under inorganic chemistry which is not true. I said that ‘a lot of the inorganic compounds that are studied are man-made’ meaning that the cutting edge of research is mostly man-made substances.
9. Apparently water is not the most inflammable substance. I thought it was so that is interesting.
10. In the bonding section, hydrogen bonding and van der waals forces are technically inter molecular forces.
Here are some of the references I used for this video if you’d like to dig a little deeper
Early smelting:
Categorisation of reactions
And The Great Courses Plus have lots of great videos:
‘The Great Courses Plus is currently available to watch through a web browser to almost anyone in the world and optimized for the US market. The Great Courses Plus is currently working to both optimise the product globally and accept credit card payments globally.’
Thanks so much to my supporters on Patreon. If you enjoy my videos and would like to help me make more this is the best way and I appreciate it very much.
Domain of Science forum:
Frontiers of Space:
Atomic Adventure:
Intergalactic Activity Book:
Solar System App:
Find me on ., instagram, and my website: